How Serum Institute’s WHO-approved malaria vaccine will change the fight against killer disease

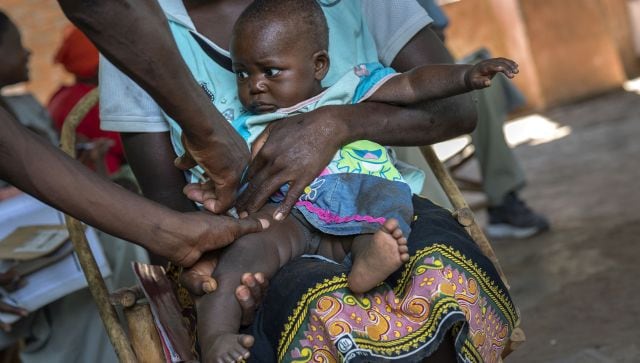
A baby from the Malawi village of Tomali is injected with the world’s first vaccine against malaria in a pilot programme. The World Health Organization authorized a second malaria vaccine on Monday, a decision that could offer countries a cheaper and more effective option than the world’s first shot against the parasitic disease. AP
India is making big strides in providing vaccines to the world. During the COVID-19 pandemic, India-made jabs offered support to hundreds of nations. Leading from the front was the Pune-based Serum Insitute of India, the world’s largest vaccine manufacturer. It supplied the “world’s least expensive and WHO-accredited vaccines to as many as 170 countries”. Now it joining the big fight against malaria.
On Monday, the World Health Organization (WHO) recommended the use of the R21/Matrix-M™ malaria vaccine developed by the University of Oxford and the Serum Institute of India (SII). Only the second malaria vaccine to be developed, its low cost and massive production will help curb the mosquito-borne disease considered to be fatal, killing mostly children.
What is the R21/Matrix-M™ malaria vaccine?
The WHO recommended the vaccine after it met the required safety, quality and effectiveness standards. It is developed by the Jenner Institute at Oxford University and Serum Institute of India with support from the European and Developing Countries Clinical Trials Partnership (‘EDCTP’), the Wellcome Trust, and the European Investment Bank.
The health agency is now “reviewing the vaccine for prequalification, which is the WHO stamp of approval, and will enable GAVI (a global vaccine alliance) and UNICEF to buy the vaccine from manufacturers,” WHO chief Tedros Adhanom Ghebreyesus told a media briefing in Geneva on Monday.
In an official statement, SII CEO Adar Poonawalla, said, “For far too long, malaria has threatened the lives of billions of people across the globe, disproportionately affecting the most vulnerable amongst us. This is why the WHO recommendation and approval of the R21/Matrix-M™ vaccine marks a huge milestone on our journey to combat this life-threatening disease, showing what exactly can be achieved when the public and private sector, scientists and researchers, all work together towards a shared goal.”

When will the vaccine be available?
R21will become available by mid-2024. The doses would cost between $2 (Rs 166) and $4 (Rs 333). There are four doses needed per person. The price of this is about half of the first malaria vaccine, RTS,S.
The new vaccine will be sold under the brand Mosquirix. It has been approved for use in Burkina Faso, Ghana, and Nigeria.
Dr Tedros said, “I used to dream of the day we would have a safe and effective vaccine against malaria, now we have two.”
Also read: Why malaria is the next biggest cause of child mortality among other causes
How are the two vaccines different?
The first vaccine was backed by WHO in 2021 to prevent malaria among children. RTS,S is developed by British pharmaceutical giant GSK.
According to the WHO, the effectiveness of both vaccines is “very similar” and there is no evidence to prove that one is better than the other.
The big difference is the ability to manufacture the R21 vaccine. The Serum Institute of India has already established production capacity for 100 million doses per annum, which will be doubled over the next two years, the company said in a statement.
There are 18 million doses of RTS,S so far, reports the BBC.
The two vaccines use similar technologies and target the same stage of the malaria parasite’s lifecycle. However, the SII vaccine is easier to produce as it requires a smaller dose and uses a simpler adjuvant, a chemical given in the vaccine that jolts the immune system into action, the BBC report says.
Both vaccines have similar efficacy rates of around 75 per cent when administered under the same conditions.

Why is the second malaria vaccine a big deal?
A second malaria vaccine was the need of the hour. “Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster and to bring us closer to our vision of a malaria-free future,” the WHO chief said.
Prof Sir Adrian Hill, director of the Jenner Institute in Oxford where R21 was developed, is quoted by the BBC as saying, “The vaccine is easily deployable, cost-effective and affordable, ready for distribution in areas where it is needed most, with the potential to save hundreds of thousands of lives a year.”
Half of the world’s population lives in a malaria high-risk area and a majority of cases and deaths are reported from the African continent.
Dr Matshidiso, the WHO regional director for Africa, said the vaccine developed by SII and Oxford held great potential for the continent and helped close the huge demand-and-supply gap. “Delivered to scale and rolled out widely, the two vaccines can help bolster malaria prevention and control efforts and save hundreds of thousands of young lives in Africa from this deadly disease,” Moeti told news agency AFP.
Also read: Explained: How scientists engineered mosquitoes that cannot spread malaria
How many lives are affected by malaria every year?
In 2021, the world reported 247 million cases of malaria. The disease claimed 619,000 lives, most of them children under the age of five.
More than 95 per cent of malaria cases are found in Africa.
With inputs from agencies
For all the latest health News Click Here