FDA panel sets June 15 meeting on Pfizer, Moderna Covid vaccines for infants and toddlers
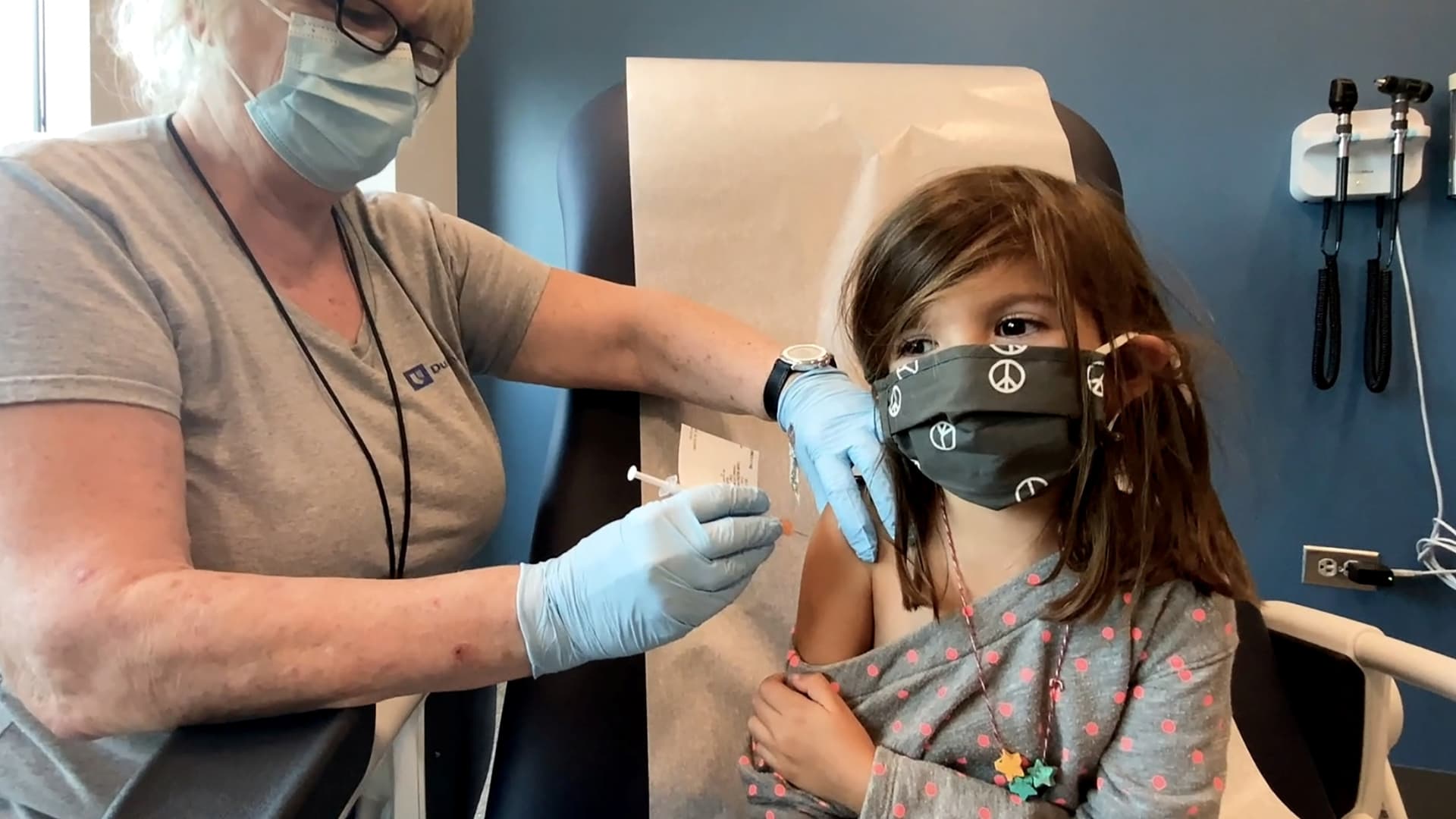
Bridgette Melo, 5, prepares for her inoculation of one of two reduced 10 ug doses of the Pfizer BioNtech COVID-19 vaccine during a trial at Duke University in Durham, North Carolina September 28, 2021 in a still image from video.
Shawn Rocco | Duke University | via Reuters
The Food and Drug Administration’s expert committee will meet June 15 to review new data on Pfizer’s and Moderna’s Covid vaccines for infants and toddlers, setting the stage for the shots to receive emergency use authorization early this summer.
“We know parents are anxious for us to determine if these vaccines are safe & effective,” the FDA said in a post Monday on Twitter. “We are working as quickly as possible to carefully review all the data.”
The FDA’s committee of independent experts will review the safety and efficacy data of the vaccines in an open public meeting and make a recommendation on whether the agency should authorize the shots. The FDA is not obligated to follow the committee’s recommendation, though it usually does.
The FDA announced the June date hours after Pfizer and BioNTech said their three-dose vaccine for children ages 6 months to 5 years old was 80% effective at preventing illness from the omicron Covid variant based on preliminary data from a clinical trial.
The FDA and Pfizer had originally sought to fast-track authorization of the first two doses in February during the winter omicron wave. However, Pfizer decided to delay its application to wait on data from the third shot after the first two doses only had 30% to 40% effectiveness against omicron.
Moderna asked the FDA to authorize its two-dose vaccine for kids under age 6 in April. The two-shot vaccine was about 51% effective against infection from omicron in children under age 2, and about 37% among kids age 2 to 5 years old. However, Moderna Chief Medical Officer Dr. Paul Burton said the antibody levels observed in the children should translate to high levels of protection against severe illness.
The FDA said it will complete its review of Pfizer’s and Moderna’s applications within days of each other, which means both vaccines would receive authorization at about the same time if the agency believes the data supports such a decision, giving parents two options to choose from.
Moderna’s vaccine for infants and toddlers consists of two 25-microgram shots, while Pfizer is using a triple course of 3-microgram shots. The dosage for both vaccines is much smaller than what the companies use for adults.
Children under age 5 are the only group in the U.S. that is not currently eligible for vaccination against Covid. Many parents and physicians have been calling on the FDA to authorize the shots for months. As omicron swept through communities over the winter, it also infected large numbers of children. The Centers for Disease Control and Prevention estimates that about 75% of children ages 11 and under had been infected with Covid as of February.
Though Covid is normally less severe in children than adults, hospitalizations of kids under age 5 were five times higher during the omicron wave than the previous delta surge, according to the CDC. Public health experts are also worried children developing long Covid and multisystem inflammatory syndrome, or MIS-C for short, a condition associated with viral infection.
Covid infections are rising again in the U.S. as even more transmissible omicron subvariants sweep the country. The U.S. is reporting more than 105,000 Covid cases per day on average as of Friday, a 16% increase over the week prior, according to CDC data. Hospitalizations have also risen 16% over the past week, with more than 3,300 patients admitted with Covid per day on average, according to the data.
For all the latest health News Click Here